A Liquid Unknown Was Found to Be Insoluble in Water
This error leads to the increased density values. The unknown was found to have a boiling point of 107C at 658 mmHg.

Solved Point Of Liquid Unknown D Boiling Estimated True Chegg Com
A liquid unknown was found to be insoluble in water and soluble in cyclohexane and alcohol boiling point of 58C at 670 mm Hg.

. If the unknown liquid was insoluble in water would it float on top of the water or would it sink to the bottom under the water. A liquid unknown was found to be insoluble in water andsoluble in cyclohexane and alcohol. A liquid unknown was found to be insoluble in water andsoluble in cyclohexane and alcohol.
Tap the tube with your finger to mix or stir gently with a glass stirring rod. A Malic acid in water. The unknown was found to have a boiling point of 145C at 658 mm Hg 760 mm Hg normal.
What could you do to confirm your answer. The unknown was found to have a boiling point of 107C at 658 mmHg. As per the given data The unknown liquid is insoluble in water and soluble in cyclohexane and alcohol.
What could you do to confirm answer. For each of the following pairs of solutes and solvent predict whether the solute would be soluble or insoluble. Note that the solution containing Pb 2 clears.
We review their content and use your feedback to keep the quality high. The liquid unknown is Chloroform. The volume would be more than what it should actually be and it would cause the density calculation to be too small.
The unknown was found to have a boiling point of 58C at 670 mmHg. D 9091 gmL. A unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 C at 658 mm Hg.
Experts are tested by Chegg as specialists in their subject area. Centrifuge decant and discard the supernatant liquids. A liquid unknown is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 145 C at 658 mm Hg.
The container in which it is kept must be pre-weighed and the difference of the total and the container the liquids weight. What is the substance. The precipitate turned grey-black upon addition of 6M NH 3The student concluded that the unknown contained Hg 2 2 ion.
What is the substance. Question a liquid unknown was found to be insoluble in water andsoluble in cyclohexane and alcohol. A liquid was found to be insoluble in water and soluble in cyclohexane and alcohol.
A liquid unknown was found to be insoluble in water and soluble in cyclohexane and. Answer 1 of 2. What is the substance.
What is the substance. Float A student received an unknown solid sample and determined the mass of the solid to be 5796 g. What is the substance.
Since density and volume are inversely related air bubbles would result in a lower density. You will need to become familiar with the solubility rules because that will help you determine what compounds are soluble and what compounds are insoluble in water. Also test the pH of water as a.
Wash the precipitates by adding 2 drops of distilled water to each tube. Add 1 drops of a liquid sample or about 25 mg of a solid sample to 05 mL of distilled or deionized water in a test tube. The unknown was found to have aboiling point of 58C at 670 mm Hg.
A liquid unknown was found to be insoluble in water and soluble in cyclohexane and alcohol. What could you do to confirm your answer. Solubility Technique 10 questions.
Im going to repost my answer to a similar question posed by the same OP. Chemistry questions and answers. A liquid unknown was found to be insoluble in water and soluble in cyclohexane and alcohol.
The substance is Bromoform. A liquid unknown is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 145 C at 658 mm Hg. What is the substance.
The unknown was found to have a boiling point of 58 C at 670 mm Hg. A liquid was found to be insoluble in water and soluble in cyclohexane and alcohol. If the unknown is water-soluble test the solution with pH paper.
What is the substance. The unknown was found to have aboiling point of 58C at 670 mm Hg. The unknown was found to have aboiling point of 58C at 670 mm Hg.
The air bubbles would take up more space resulting in a greater calculated volume. What is the substance. Heres what these rules look like.
What could you do. The student received a low mark on the experiment because the unknown actually contained all three Group I cations. The elevated results of density are observed as the volume gets increased.
The unknown was found to have a. See full answer below. So start with NaCl or sodium chlorideThe solubility rules tell you that all halides are soluble with the.
What could you do to confirm your answer. A liquid was found to be insoluble in water and soluble in cyclohexane and alcohol. Decant and discard the supernatant liquid from each tube.
Pb 2 aq 2Cl- aq -- PbCl 2 s 2 The precipitate was separated from the supernatant liquid in 1 and was found to be insoluble in hot water. Add 2 drops of distilled water to each tube then heat the tubes in a boiling water bath. Record the sample as soluble or insoluble.
The tube may be centifuged and. That would be copper II hydroxide or CuOH_2.
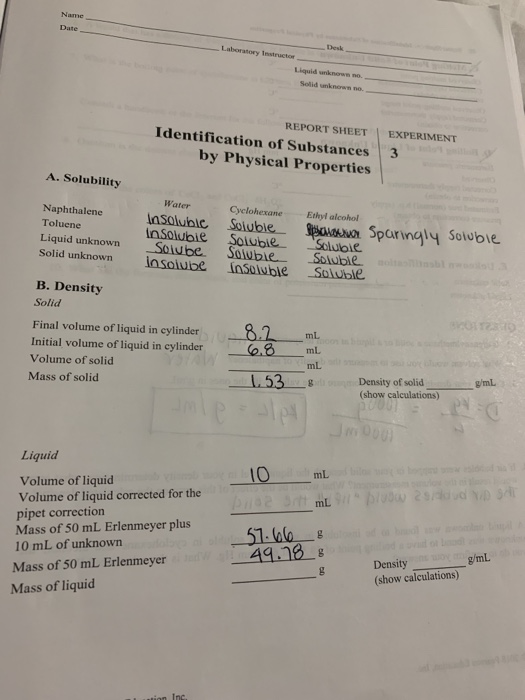
Name Date Desk Laboratory Instructor Lorem Solidnon Chegg Com

Solved 2 Suppose A Solid Unknown Is Insoluble In Water Chegg Com

7 Vitamin K Myths Busted Myth Busted Vitamin K Health Options

Solved 5 Aliquid Unknown Was Found To Be Insoluble In Water Chegg Com

Solved Explain The And How To Calculate Melting Point Chegg Com
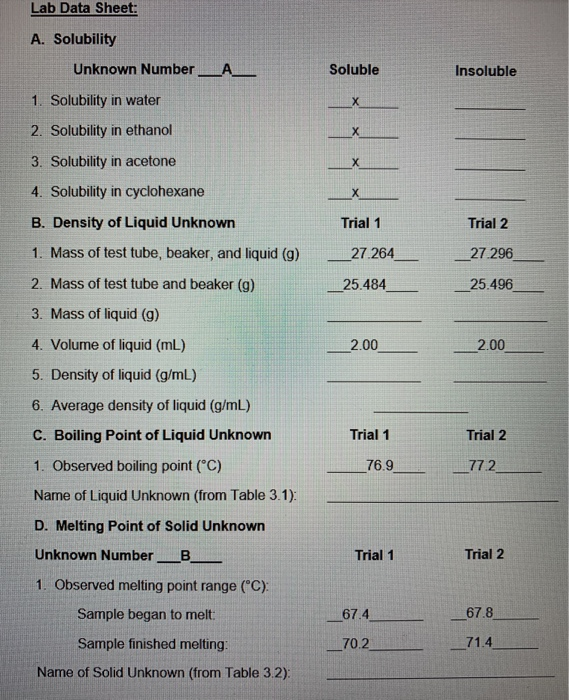
Solved Lab Data Sheet A Solubility Unknown Number A Chegg Com

5 A Liquid Unknown Was Found To Be Insoluble In Chegg Com

Standard Solution Easy Science Easy Science Solutions Science Facts
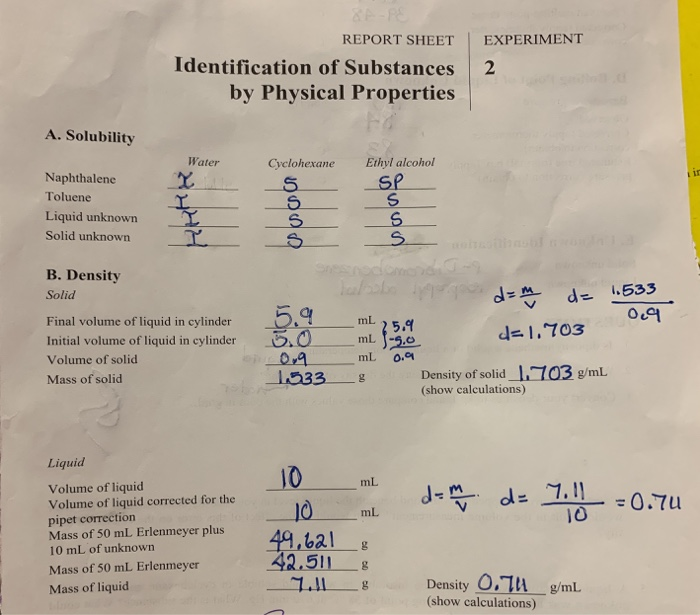
30 Report Sheet Identification Of Substances By Chegg Com

30 Report Sheet Identification Of Substances By Chegg Com

Solved Figure 3 1 Figure 3 2 Solubility Compound Density Chegg Com

Name Date Desk Laboratory Instructor Lorem Solidnon Chegg Com

Some Useful Products Are Formed From The Living Substance Of The Protoplasm During The Metabolic Activity Different Types Of Colours E Flowers Daughter Cells

16 Unknown Benefits Of Orange Oil Temel Yaglar Cil Portakal

Pb No 3 2 Aq Hcl Aq Write The Equation And The Net Ionic Equation Socratic Solubility College Chemistry Chemistry Notes
Solubility Science How Much Is Too Much Scientific American

Amongst The Following Compounds Identify Which Are Insoluble Partially Soluble And Highly Soluble In Water I Phenol Ii Toluene Iii Formic Acid Iv Ethylene Glycol V Chloroform Vi Pentanol

Solved 2 Suppose A Solid Unknown Is Insoluble In Water Chegg Com
Comments
Post a Comment